The d- and f- block Elements
Topics:The Transition Elements (d-Block)
Position in the Periodic Table
- Their position is in between s- and p- block elements.
- There are four rows of transition elements − 3d, 4d, 5d, and 6d. The fourth row of 6d is still incomplete.
Electronic Configuration
- General outer electronic configuration is (n −1)d1−10 ns1−2.
- But this generalization has several exceptions as there is very little difference in energy between (n − 1)d and ns orbitals. For example, Cr has electronic configuration 3d5 4s1instead of 3d4 4s2. Cu has electronic configuration 3d10 4s1 instead of 3d9 4s2.
- Outer electronic configurations of the transition elements in the ground state are listed in the following tables:
1st series
|
2nd series
| ||||
Element
|
Atomic number
|
Outer electronic configuration
|
Element
|
Atomic number
|
Outer electronic configuration
|
Sc
|
21
|
3d14s2
|
Y
|
39
|
4d15s2
|
Ti
|
22
|
3d24s2
|
Zr
|
40
|
4d25s2
|
V
|
23
|
3d34s2
|
Nb
|
41
|
4d45s1
|
Cr
|
24
|
3d54s1
|
Mo
|
42
|
4d55s1
|
Mn
|
25
|
3d54s2
|
Tc
|
43
|
4d65s1
|
Fe
|
26
|
3d64s2
|
Ru
|
44
|
4d75s1
|
Co
|
27
|
3d74s2
|
Rh
|
45
|
4d85s1
|
Ni
|
28
|
3d84s2
|
Pd
|
46
|
4d105s0
|
Cu
|
29
|
3d104s1
|
Ag
|
47
|
4d105s1
|
Zn
|
30
|
3d104s2
|
Cd
|
48
|
4d105s2
|
3rd series
|
4th series
| ||||
Element
|
Atomic number
|
Outer electronic configuration
|
Element
|
Atomic number
|
Outer electronic configuration
|
La
|
57
|
5d16s2
|
Ac
|
89
|
6d17s2
|
Hf
|
72
|
5d26s2
|
Rf
|
104
|
6d27s2
|
Ta
|
73
|
5d36s2
|
Db
|
105
|
6d37s2
|
W
|
74
|
5d46s2
|
Sg
|
106
|
6d47s2
|
Re
|
75
|
5d56s2
|
Bh
|
107
|
6d57s2
|
Os
|
76
|
5d66s2
|
Hs
|
108
|
6d67s2
|
Ir
|
77
|
5d76s2
|
Mt
|
109
|
6d77s2
|
Pt
|
78
|
5d96s1
|
Ds
|
110
|
6d87s2
|
Au
|
79
|
5d106s1
|
Rg
|
111
|
6d107s1
|
Hg
|
80
|
5d106s2
|
Uub
|
112
|
6d107s2
|
- Zn, Cd, and Hg are not regarded as transition elements.
- Reason − The orbitals of these elements are completely-filled. [Electronic configuration is (n− 1) d10 ns2]
- Ions of configuration d1−9 have similar magnetic and electronic properties.
- Elements with partially-filled d-orbitals exhibit certain characteristic properties such as showing a variety of oxidation states, formation of coloured ions, and formation of complex with a variety of ligands.
- They and their compounds have catalytic property and are paramagnetic in nature.
Example :
The d orbitals are completely filled for the elements copper, palladium, silver and gold in their respective series along with Zn, Cd and Hg.
Ni
|
Cu 3d10 4s 1
|
Zn 3d10 4s2
|
Pd 4d10 5s0
|
Ag 4d10 5s 1
|
Cd 4d10 5s2
|
Pt
|
Au 5d10 6s 1
|
Hg 5d10 6s2
|
Even though the ground state of the atom has a d10 configuration, Pd and the coinage metals (Cu, Ag and Au) behave as typical transition elements while Zn, Cd and Hg are not regarded as transition elements. Why?
Cu II has a d9 configuration.
Pd II and Au (III) have d8 configuration i.e. they have incomplete d orbital.
Hence, they are regarded as transition elements.
In case of Zn, Cd and Hg, the ions Zn2+, Cd2+ and Hg2+ have a d10 configuration i.e. even in oxidised states, these elements have completely filled d−orbitals. Hence, they are not regarded as transition elements.
Example :
Which of the following statements regarding transition elements is not correct?
- A )Their position in the periodic table is between s − block and p − block.
- B )Like s − block and p − block, electrons are added in the outer shell of the atom.
- C )Their general electronic configuration in ground state is (n − 1)d10ns2.
- D )They have incompletely filled d − orbitals.
In s − block and p − block elements, electrons are added to the outer shell of atoms. Thus, the electronic configurations are
But, in d − block elements (transition elements), electrons are added to the penultimate shell i.e. second last shell. The electronic configuration of iron, a d-block element is shown below.
Topics:General Properties of Transition Metals - I
Physical Properties
- Nearly all transition metals display metallic properties such as high tensile strength, ductility, malleability, high thermal and electrical conductivity and metallic lustre.
- Very hard and have low volatility (Exception: Zn, Cd and Hg)
- The melting and boiling points of the first transition series are lower than those of the heavier transition elements.
Reason: Occurrence of stronger metallic bonding (M−M bonding) in heavier metals
- Trends in melting points of transition metals are shown in the given figure.
- Transition elements have high effective nuclear charge and a large number of valence electrons. Therefore, they form very strong metallic bonds. As a result, the enthalpy of atomisation of transition metals is high.
- Trends in enthalpies of atomisation of transition elements are shown in the given figure.
- The enthalpies of atomisation of the elements in the first transition series are lower than those of the corresponding elements in the second and third transition series.
Variation in Atomic and Ionic Sizes of Transition Metals
- Atomic size generally decreases from left to right across a period.
- The atomic sizes of the elements of the first transition series are smaller than those of the corresponding heavier elements (elements of the 2nd and 3rd transition series).
- However, the atomic sizes of the elements in the third transition series are virtually the same as those of the corresponding members in the second transition series. This is due to lanthanoid contraction.
- Trends in atomic radii of transition elements are shown in the given figure.
Ionisation Enthalpies
- In each of the three transition series, the first ionisation enthalpy increases from left to right. However, there are some exceptions.
- The first ionisation enthalpies of the third transition series are higher than those of the first and second transition series.
Reason: Poor shielding effect of 4f electrons in the third transition series
- Certain elements in the second transition series have higher first ionisation enthalpies than the elements corresponding to the same vertical column in the first transition series.
- There are also elements in the 2nd transition series whose first ionisation enthalpies are lower than those of the elements corresponding to the same vertical column in the 1sttransition series.
Example :
Use the following information to answer the next question.
Account for the following:
Sc → Y → La: Size increases
Ti → Zr → Hf: First size increases and then decreases
V → Nb → Ta: First size increases and then remains same
Across the third period, atomic size increases from Sc → Y → La due to regular addition of new shells.
There is an intervention of 14 lanthanide elements between lanthanum (La) and Hafnium (Hf). Due to this, there is a gradual decrease in the size of the lanthanide elements. This is known as lanthanide contraction. Hence, the size slightly decreases from Zr → Hf and remains the same for Nb → Ta.
Example :
Which of the following transition elements has the highest third ionization enthalpy?
- A )V
- B )Cr
- C )Fe
- D )Mn
For the third ionization enthalpy, one electron has to be removed from Mn2+. The electronic configuration of Mn2+ is 3d5 , which is a half-filled configuration and hence, is extra stable. So, among all the given elements, the third ionization enthalpy for Mn is the highest.
Example :
Ram gifted a pair of gold bangles to his wife having the composition of 87.5 % gold, 5% copper, 3 % bronze and 1.5 % silver. What would be the rating of the bangles in terms of carat?
- A )21
- B )18
- C )15
- D )24
Pure gold = 24 carat
87.5% gold = 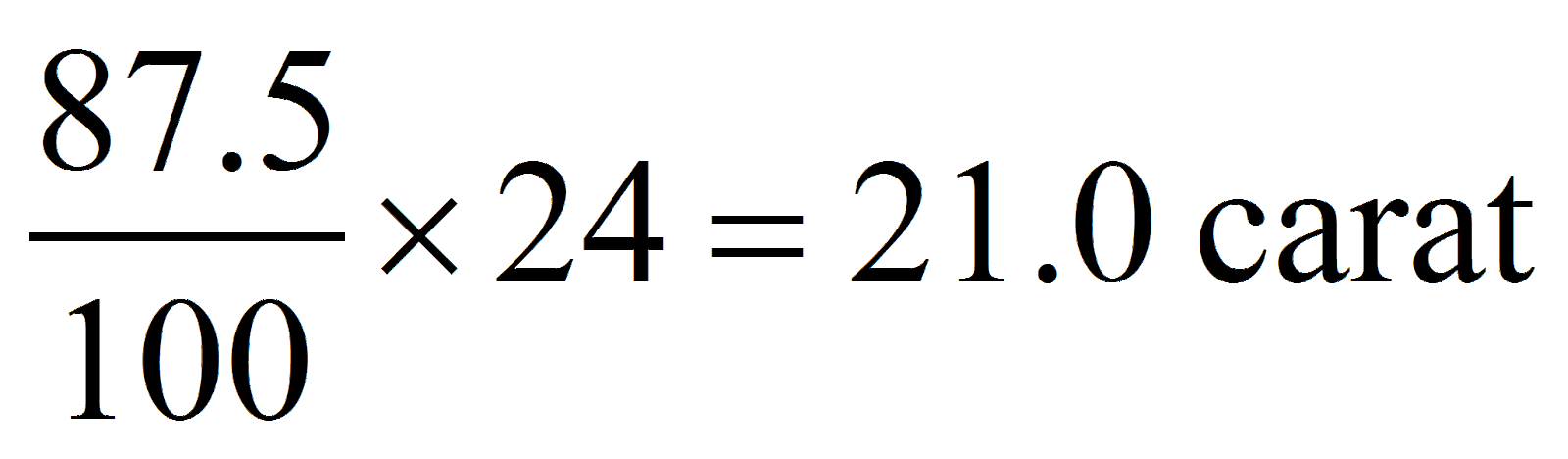
Hence, option (a) is correct.
Topics:General Properties of Transition Metals - II
Oxidation States
- All transition metals exhibit a variety of oxidation states.
- The oxidation states of the first row transition metals are listed in the given table.
Sc
|
Ti
|
V
|
Cr
|
Mn
|
Fe
|
Co
|
Ni
|
Cu
|
Zn
|
-
|
+2
|
+2
|
+2
|
+2
|
+2
|
+2
|
+2
|
+1
|
+2
|
+3
|
+3
|
+3
|
+3
|
+3
|
+3
|
+3
|
+3
|
+2
|
-
|
-
|
+4
|
+4
|
+4
|
+4
|
+4
|
+4
|
+4
|
-
|
-
|
-
|
-
|
+5
|
+5
|
+5
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
+6
|
+6
|
+6
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
+7
|
-
|
-
|
-
|
-
|
-
|
(The most common ones are in bold types)
- The variable oxidation states of transition elements are due to the participation of ns and (n−1)d-electrons in bonding.
- Lower oxidation state is exhibited when ns-electrons take part in bonding.
- Higher oxidation states are exhibited when (n − 1)d-electrons take part in bonding.
- In each group, the highest oxidation state increases with the increase in atomic number; reaches the maximum in the middle, and then starts decreasing.
Example: In the first transition series, manganese has the maximum oxidation state (+7).
- For the elements of the first transition series, the most common oxidation state is +2. (Exception: Scandium)
- The highest oxidation state shown by any transition metal is +8. (Example: osmium and ruthenium)
- Some transition elements show zero oxidation state in their compounds.
Trends in the M2+/M Standard Electrode Potentials
- The observed and calculated values of standard electrode potential (Eθ) for the elements Ti to Zn are shown in the given figure.
- The sum of enthalpies of sublimation and ionization is not balanced by hydration enthalpy in case of copper. Hence, the Eθ(M2+/M) value for copper is positive.
- The values of Eθ for Mn, Ni, Zn are more negative than the expected trend.
- Reason: The stability of the half-filled d-subshell in Mn2+ and the completely filled d10configuration in Zn2+
Negative value of Eθ for Ni is due to the highest negative hydration energy.
Trends in the M3+/M2+ Standard Electrode Potentials
- The given table lists the values of Eθ for M2+/M and M3+/M2+.
Element
|
-
|
Sc
|
Ti
|
V
|
Cr
|
Mn
|
Fe
|
Co
|
Ni
|
Cu
|
Zn
|
Standard
electrode
|
M2+/M
|
-
|
-1.63
|
-1.18
|
-0.90
|
-1.18
|
-0.44
|
-0.28
|
-0.25
|
+0.34
|
-0.76
|
Potential
EΘ/ V
|
M3+/M2+
|
-
|
-0.37
|
-0.26
|
-0.41
|
+1.57
|
+0.77
|
+1.97
|
-
|
-
|
-
|
- The highest value for Zn is due to the formation of the stable d10 configuration of Zn2+.
- Similarly, comparatively higher value for Mn is due to the formation of the stable d5configuration of Mn2+.
Chemical Reactivity
- Many of the transition metals are electropositive. Hence, they dissolve in mineral acids.
- Few of them are ‘noble’, and are unaffected by simple acids.
- Metals of the first series are relatively more reactive and are oxidised by 1M H+.
- The Eθ values for M2+/M indicate the decreasing tendency to form divalent ions across the series.
- The Eθ values for M3+/M2+ show that Mn3+ and Co3+ ions are the strongest oxidising agents in aqueous solution.
- Ti2+, V2+ and Cr2+ are strong reducing agents, and will liberate hydrogen gas from dilute acids.
Example :
Which of the following pairs of compounds shows zero-valent state?
- A )[Fe (CO)4]2+ and [Fe (CO)5]
- B )[Fe (CO)5] and [Ni (CO)4]
- C )[Ni2 (CO)6]2 + and [Ni(CO)4]
- D )[Ni2 (CO)6)2 + and [Fe(CO)4]2+
[Fe (CO)4]2+ : Oxidation state of Fe is + 2
[Ni2 (CO)6]2 + : Oxidation state of Ni is +2.
Example :
In which of the following pairs of ions is the lower oxidation state more stable in aqueous solution?
- A )Cu+, Cu2+
- B )V2+, V4+
- C )Tl+, Tl3+
- D )Cr2+, Cr3+
Tl is the last element of group 13. Tl+ is more stable than Tl3+ due to inert pair effect.
All other pairs of ions have the higher oxidation states more stable. This is due to the high hydration enthalpy (more negative ΔHydH) which compensates the ionization enthalpy.
Example :
When KMnO4 acts as an oxidizing agent and ultimately forms MnO2, Mn(+2), Mn(OH)3 and MnO4(-2), then the number of electrons transferred in each case respectively is:
- A )3,5,4,1
- B )4,3,1,5
- C )1,3,4,5
- D )5,4,3,1
KMnO4 MnO2 Mn(+2) Mn(OH)3 MnO4(-2)
Oxidation state of Mn +7 +4 +2 +3 +6
Electrons added 0 3 5 4 1
Hence, option (a) is correct.
Example :
Madhav during an inorganic experiment treated magnesium ribbon with sulphuric acid and observed an evolution of a gas. He stored the gas in a flask having a capillary tube. To predict the nature of the gas, he brought a lighted matchstick near the mouth of the capillary tube and observed that the matchstick went off followed by a pop sound. He concluded it to be hydrogen. When he treated the magnesium ribbon with conc. HCl, no characteristic observation was made. This is probably due to:
- A )NO−3 ion is reduced in preference to hydronium ion
- B )Conc. HNO3 is a weaker acid than conc. H2SO4 and conc. HCl
- C )Conc. HNO3 acts as a reducing agent
- D )Magnesium is more reactive than H2
NO−3 ion is reduced to NO2 in preference to H3O+ ion.
Hence, option (d) is correct.
Topics:General Properties of Transition Metals -III
Magnetic Properties
- Diamagnetic substance − Repelled by the applied field
Paramagnetic substance − Attracted by the applied field
- Ferromagnetic substance − Attracted very strongly
- Paramagnetism arises due to the presence of unpaired electrons.
- Magnetic moment can be calculated by using ‘spin-only’ formula, i.e.,
Where,
n = Number of unpaired electrons
μ = Magnetic moment in Bohr magneton (BM)
- Magnetic moment increases with the increase in the number of unpaired electrons.
- Magnetic moments (calculated from ‘spin-only’ formula) for some ions of the first row transition elements are listed in the given table.
Ion
|
Configuration
|
Unpaired
electron(s)
|
Magnetic moment (BM)
| |
Calculated
|
Observed
| |||
Sc3+
|
3d0
|
0
|
0
|
0
|
Ti3+
|
3d1
|
1
|
1.73
|
1.75
|
Tl2+
|
3d2
|
2
|
2.84
|
2.76
|
V2+
|
3d3
|
3
|
3.87
|
3.86
|
Cr2+
|
3d4
|
4
|
4.90
|
4.80
|
Mn2+
|
3d5
|
5
|
5.92
|
5.96
|
Fe2+
|
3d6
|
4
|
4.90
|
5.3 − 5.5
|
Co2+
|
3d7
|
3
|
3.87
|
4.4 − 5.2
|
Ni2+
|
3d8
|
2
|
2.84
|
2.9 − 3, 4
|
Cu2+
|
3d9
|
1
|
1.73
|
1.8 − 2.2
|
Zn2+
|
3d10
|
0
|
0
|
-
|
Formation of Coloured Ions
- An electron from a lower energy d-orbital is excited to a higher energy d-orbital when the energy of excitation corresponds to the frequency of light absorbed.
- This frequency of light generally lies in the visible region.
- The colour observed is the complementary colour of the light absorbed.
- Colours of some of the first row transition metal ions (aquated) are listed in the given table
Configuration
|
Example
|
Colour
|
3d0
|
Sc3+
|
Colourless
|
3d0
|
Ti4+
|
Colourless
|
3d1
|
Ti3+
|
Purple
|
3d1
|
V4+
|
Blue
|
3d2
|
V3+
|
Green
|
3d3
|
V2+
|
Violet
|
3d3
|
Cr3+
|
Violet
|
3d4
|
Mn3+
|
Violet
|
3d4
|
Cr2+
|
Blue
|
3d5
|
Mn2+
|
Pink
|
3d5
|
Fe3+
|
Yellow
|
3d6
|
Fe2+
|
Green
|
3d6, 3d7
|
Co3+, Co2+
|
Blue, pink
|
3d8
|
Ni2+
|
Green
|
3d9
|
Cu2+
|
Blue
|
3d10
|
Zn2+
|
Colourless
|
Formation of Complex Compounds
- Transition metals form a large number of complex compounds.
- Reason: Comparatively smaller size of metal ions, high ionic charges and availability of d-orbitals for bond formation
- Example:
, etc.
Catalytic Properties
- Transition metals and their compounds are known for their catalytic activity.
- Example: Vanadium (V) oxide (in Contact Process), nickel (in Catalytic Hydrogenation), etc.
- Iron (III) catalyses the reaction of iodide with persulphate ions.
- Mechanism:
Formation of Interstitial Compounds
- Interstitialcompounds
- Formed when small atoms like H, C, N are trapped inside the crystal lattices of metals
- Usually non-stoichiometric
- Neither typically ionic nor covalent
- Example: TiC, Mn4N, Fe3H, etc.
- Characteristics of interstitial compounds
- Higher melting points than pure metals
- Very hard
- Retain metallic conductivity
- Chemically inert
Alloy Formation
- Alloys are readily formed by these metals.
- Reason: Because of similar radii and other characteristics of transition metals
- Alloys, so formed, are hard and have high melting points.
- Ferrous alloys − Cr, V, W, Mo and Mn are used for the production of a variety of steels and stainless steel.
- Alloys of transition metals with non-transition metals: Brass (Cu − Zn) and bronze (Cu − Sn)
Example :
The magnetic moment of Zn2+, Cu2+, Sc3+ and Ti3+ ions are
The spin only formula is
μ = magnetic moment
n = Number of unpaired electrons
For Zn2+, Cu2,Sc3+ and Ti3+ the values of n are 0, 1, 0, 1
Example :
Which of the following phenomena is responsible for colour in an aqueous solution of KMnO4?
- A )
Charge transfer
- B )
f − f transition
- C )
d − d transition
- D )
d − f transition
Oxidation state of Mn in KMnO4 is +7. Its electronic configuration is d0. Since there is no d − electron, it cannot show d − d transition. Hence, the colour of KMnO4 is due to charge transfer.
In 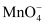 , oxygen behaves as a ligand that transfers its electron from p-orbital to empty d-orbital of Mn ion. Transferring an electron means transferring a charge, which results in charge transfer spectra. Due to this charge transfer spectra, an aqueous solution of KMnO4 is coloured.
, oxygen behaves as a ligand that transfers its electron from p-orbital to empty d-orbital of Mn ion. Transferring an electron means transferring a charge, which results in charge transfer spectra. Due to this charge transfer spectra, an aqueous solution of KMnO4 is coloured.
Example :
Match the following complexes with the appropriate metal found in them:
- A )
Chlorophyll (i) Fe
- B )
Vitamin B12 (ii) Zn
- C )
Insulin (iii) Mg
- D )
Haemoglobin (iv) Co
(a) (A)—ii ,(B)---i ,(C)---iv, (D)---iii
(b) (A)---iv , (B)---ii , (C)---iii, (D)---i
(c) (A)---iii , (B)---i , (C)---ii , (D)---iv
(d) (A)---iii , (B)---iv, (C)---ii , (D)---i
Insulin contains zinc, haemoglobin contains iron, vitamin B12 contains cobalt, and chlorophyll contains magnesium.
Hence, option (d) is correct.
Example :
Which of the following electronic configurations has a magnetic moment of 5.9 B.M?
- A )3d2
- B )3d7
- C )3d9
- D )3d5
In 3d5 configuration, all the d-orbitals are singly occupied. i.e., it has maximum number of unpaired electrons.
Hence, option (d) is correct.
Topics:Some Important Compounds of Transition Elements
Oxides and Oxoanions of Metals
- Oxides are generally formed by the reaction of metals with oxygen at higher temperatures.
- All the metals (except scandium) form MO oxides, which are ionic in nature.
- As the oxidation number of a metal increases, ionic character decreases.
Potassium Dichromate ( )
)
- Prepared from chromite ore
in the following steps:
- Preparation of sodium chromate
- Conversion of sodium chromate into sodium dichromate
- Conversion of sodium dichromate to potassium dichromate
- Potassium dichromate being less soluble than sodium dichromate can be obtained in the form of orange coloured crystals by treating sodium dichromate with potassium chloride and can be removed by filtration.
- The dichromate ion
exists in equilibrium with chromate
ion at pH = 4. However, by changing the pH, they can be inter-converted.
acts as a very strong oxidising agent in the acidic medium.
takes up electrons to get reduced and acts as an oxidising agent.
- Examples:
- Oxidises iodide to iodine
- Oxidises iron (II) solution to iron (III) solution i.e., ferrous ions to ferric ions
- Oxidises H2S to sulphur
Potassium Permanganate
- It can be prepared from pyrolusite (MnO2). The ore is fused with KOH in the presence of either atmospheric oxygen or an oxidising agent such as KNO3 or KClO4 to give K2MnO4.
- The green mass can be extracted with water and then oxidised either electrolytically or by passing chlorine/ozone into the solution.
- Electrolytic oxidation:
- At anode, manganate ions are oxidised to permanganate ions.
- Oxidation by chlorine:
- Oxidation by ozone:
- Acidified KMnO4 solution oxidises Fe (II) ions to Fe (III) ions i.e., ferrous ions to ferric ions.
- Acidified potassium permanganate oxidises SO2 to sulphuric acid.
- Acidified potassium permanganate oxidises oxalic acid to carbon dioxide.
Example :
I− on oxidation by 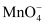 in alkaline and acidic medium gives
in alkaline and acidic medium gives
- A )
and
respectively
- B )I2 and
respectively
- C )
and I2 respectively
- D )
and I2 respectively
In alkaline medium
Iodide I− → Iodate 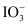
In acidic medium
Iodide I− → Iodine I2
Example :
A metal which is not affected by conc.H2SO4, HNO3 or alkalies forms a compound X .This compound X can be used to give a complex which finds its application for toning in photography. The metal is:
- A )Ag
- B )Hg
- C )Au
- D )Cu
Au is not affected by concentrated acids and strong alkalis. However, it dissolves in aqua-regia forming AuCl3which is used for toning in photography.
Hence, option (c) is correct.
Example :
In a inorganic experiment, potassium dichromate was treated with SnCl2. As soon as the reaction got over, the solution turned green. If 1 mole of SnCl2 was used, find out how many number of moles of potassium dichromate were reduced?
- A )1/3
- B )3
- C )1/6
- D )6
3 moles of Sn2+ ions reduce = 1 mole of 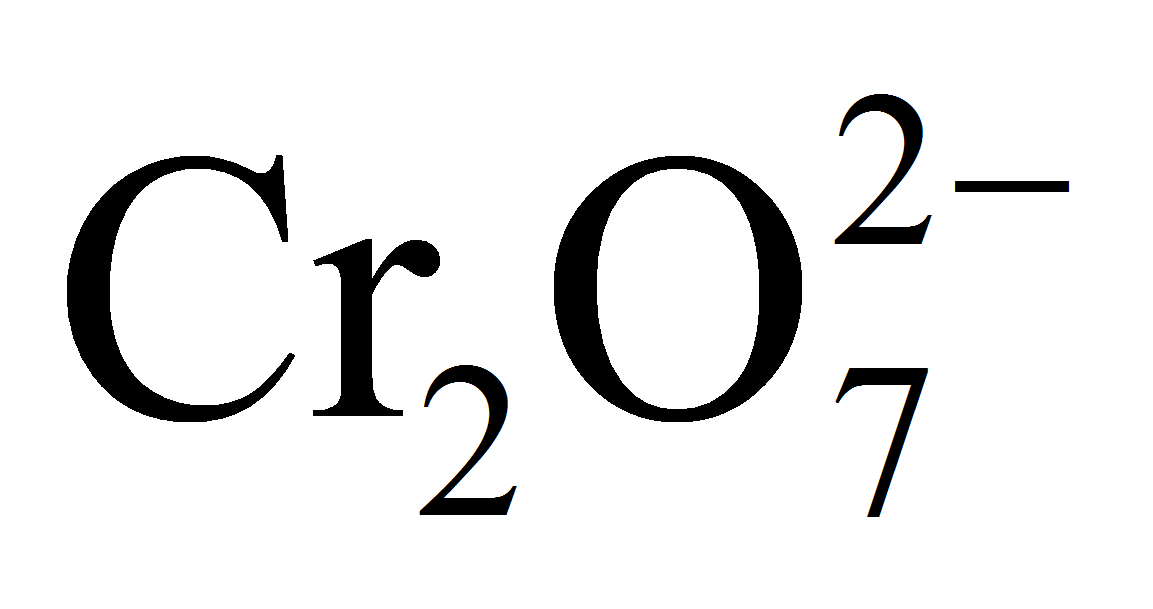
= 1 mole of K2Cr2O7
1 mole of Sn2+ ions will reduce = 1/3 mole of K2Cr2O7
Hence, option (a) is correct.
Example :
Fill in the blank with appropriate option so that the given statement is true:
Each chromium atom in potassium dichromate molecule is linked to ________ oxygen atoms.
- A )Two
- B )Three
- C )Four
- D )Five
The structure of 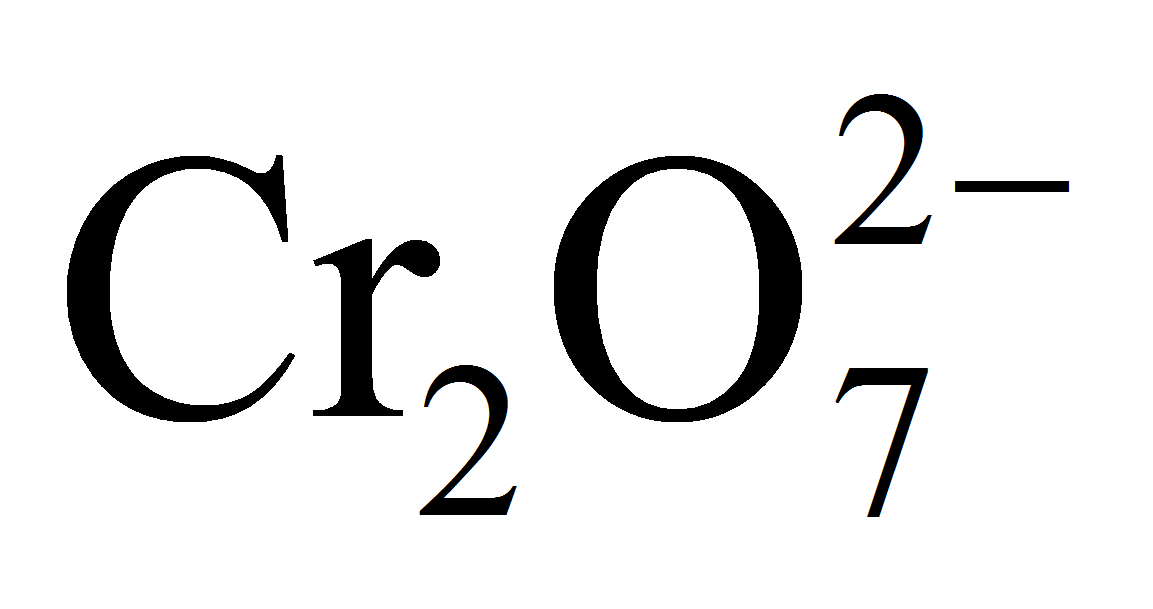 ion is:
ion is:
Hence, option (c) is correct.
Example :
Which of the following chemical reagents can be used to detect the presence of sugar in a sample?
- A )Fehling’s solution
- B )Benedict’s solution
- C )Tollen’s reagent
- D )All the above
Fehling’s solution - Cu (II) ion (complexed with tartrate ion) is heated in basic solution with a reducing sugar, and a brick red precipitate of Cu2O is formed. Mixture of alk. CuSO4 and sodium potassium tartrate is called Fehling’s solution.
Benedict’s solution - It contains Cu(II) ion complexed to citrate ion. When it is heated with reducing sugar, Cu(II) is reduced to Cu2O.
Tollen’s reagent - Ammonical AgNO3 solution is called Tollen’s reagent. Reducing sugars give silver mirror test with Tollens reagent.
Hence, option (d) is correct.
Example :
An inorganic compound (X) used in the manufacturing of ink and hair dyes gives different coloured precipitates on treatment with anions like Cl-,Br-,I-,S2-, etc. Also when this compound (X) reacts with water, it gives a residue along with nascent oxygen as one of the byproducts which is used to oxidize organic matter. Identify the compound (X).
- A )
Pb(NO3)2
- B )
Ba(NO)2
- C )
AgNO3
- D )
none of these
2AgNO3 + H2O → 2Ag + 2HNO3 + [O] → oxidises organic matter
Hence, option (c) is correct.
Topics:Lanthanoids
- Lanthanoids are the fourteen elements from lanthanum (La) to lutetium (Lu).
Electronic Configuration
- In general, the outermost electronic configuration is 4f1−146s2.
- Electronic configurations of lanthanum and lanthanoids are listed in the table
AtomicNumber
|
Name
|
Symbol
|
Electronic configurations
|
Radii/pm
| ||||
Ln
|
Ln2+
|
Ln3+
|
Ln4+
|
Ln
|
Ln3+
| |||
57
|
Lanthanum
|
La
|
5d16s2
|
5d1
|
4f0
|
-
|
187
|
106
|
58
|
Cerium
|
Ce
|
4f15d16s2
|
4f2
|
4f1
|
4f0
|
183
|
103
|
59
|
Praseodymium
|
Pr
|
4f36s2
|
4f3
|
4f2
|
4f1
|
182
|
101
|
60
|
Neodymium
|
Nd
|
4f46s2
|
4f4
|
4f3
|
4f2
|
181
|
99
|
61
|
Promethium
|
Pm
|
4f56s2
|
4f5
|
4f4
|
-
|
181
|
98
|
62
|
Samarium
|
Sm
|
4f66s2
|
4f6
|
4f5
|
-
|
180
|
96
|
63
|
Europium
|
Eu
|
4f76s2
|
4f7
|
4f6
|
-
|
199
|
95
|
64
|
Gadolinium
|
Gd
|
4f75d16s2
|
4f35d1
|
4f7
|
-
|
180
|
94
|
65
|
Terbium
|
Tb
|
4f96s2
|
4f9
|
4f8
|
4f7
|
178
|
92
|
66
|
Dysprosium
|
Dy
|
4f106s2
|
4f10
|
4f9
|
4f8
|
177
|
91
|
67
|
Holmium
|
Ho
|
4f116s2
|
4f11
|
4f10
|
-
|
176
|
89
|
68
|
Erbium
|
Er
|
4f126s2
|
4f12
|
4f11
|
-
|
175
|
88
|
69
|
Thulium
|
Tm
|
4f136s2
|
4f13
|
4f12
|
-
|
174
|
87
|
70
|
Ytterbium
|
Yb
|
4f146s2
|
4f14
|
4f13
|
-
|
173
|
86
|
71
|
Lutetium
|
Lu
|
4f145d16s2
|
4f145d1
|
4f14
|
-
|
-
|
-
|
Atomic and ionic sizes
- There is a gradual decrease in atomic and ionic radii of Lanthanoids with an increase in atomic number. This is known as lanthanoid contraction.
- Due to lanthanoid contraction, the radii of the elements of the 3rd transition series are very similar to those of the corresponding elements of the 2nd series.
Oxidation states
- The lanthanoids exhibit mainly +3 oxidation state. However, sometimes, +2 and +4 oxidation states are also exhibited.
General Characteristics
- Silvery-white, soft metals; tarnish rapidly in air
- Hardness increases with increasing atomic number
- Good conductors of heat and electricity
- 1st and 2nd ionisation enthalpies around 600 kJ mol−1 and 1200 kJmol−1 respectively
- Chemical reactions:
Uses
- In the production of alloy steels for plates and pipes
- Mixed oxides of lanthanoids are used as catalysts in petroleum cracking.
- Some lanthanum oxides are used as phosphors in television screens and other fluorescing surfaces.
Example :
Which of the following statements is not correct?
- A )La is a transition element and not a lanthanide.
- B )Zr and Hf have nearly same atomic radii.
- C )Lu(OH)3 is more basic than La (OH)3.
- D )Ionic radius decreases from La3+ to Lu3+.
The ionic sizes of lanthanides decrease from La3+ to Lu3+. According to Fajan’s rule, small sized cation Lu3+ shows high covalent character and hence basic strength of its hydroxides decrease.
Example :
Which of the following statements is not correct regarding the element cerium of the lanthanide series?
- A )+ 3 and + 4 are common oxidation states.
- B )+ 3 oxidation state is more stable than + 4.
- C )+ 4 oxidation state does not exist in solution.
- D )+ 4 oxidation state acts as an oxidizing agent.
Ce4+ exists in solution. + 4 oxidation state of Ce in solution is favoured by its noble gas configuration.
Topics:The Actinoids & Some Applications of d-and f-block Elements
Actinoids
- These include fourteen elements after actinium (from thorium to lawrencium).
- These are the radioactive elements.
Electronic Configuration:
- They have the electronic configuration of 7s2 with variation of occupancy in 5f and 6dorbitals.
- There is a gradual decrease in atomic and ionic radii of actinoids with increase in atomic number.
This is known as actinoid contraction.
Oxidation States:
- Greater range of oxidation states
- Reason: 5f, 6d, and 7s subshells are of comparable energies.
- Exhibit mainly +3 oxidation state. However, +4, +5, +6, and +7 oxidation states are also exhibited.
- Oxidation states of actinium and actinoids are given in the following table.
Ac
|
Th
|
Pa
|
U
|
Np
|
Pu
|
Am
|
Cm
|
Bk
|
Cf
|
Es
|
Fm
|
Md
|
No
|
Lr
|
3
|
3
|
3
|
3
|
3
|
3
|
3
|
3
|
3
|
3
|
3
|
3
|
3
|
3
| |
4
|
4
|
4
|
4
|
4
|
4
|
4
|
4
| |||||||
5
|
5
|
5
|
5
|
5
| ||||||||||
6
|
6
|
6
|
6
| |||||||||||
7
|
7
|
General Characteristics and Comparison with Lanthanoids
- Silvery metals
- Have a variety of structures
- Highly reactive (especially when finely divided)
- Give a mixture of oxide and hydride when treated with boiling water
- Combine with most non-metals at moderate temperatures
- React readily with hydrochloric acid, but slowly with nitric acid due to the formation of protective oxide film
- Do not react with alkalies
- Paramagnetic in nature
Their magnetic properties are complex in comparison to lanthanoids.
- Lower ionisation enthalpies than lanthanoids
- Reason − 5f electrons are less effectively shielded than 4f electrons.
Some Applications of d-and f-block Elements
- Iron is used in the construction materials.
- Fe, Cr, Ni, and Mn are used in the manufacture of steel, which is used in construction.
- TiO is used in pigment industry whereas MnO2, Zn, and Ni/Cd are used in battery industry.
- Ag, Au, Cu, and Ni are used in making coins.
- Some metals and/or their compounds (V, Ti, Al, Fe, Ni, and Pd) are used as catalysts.
- AgBr is used in photographic industry as it has special light sensitive properties.
Example :
Actinoid ions containing no 5f − electrons or seven 5f − electrons respectively are
- A )coloured and colourless
- B )colourless and coloured
- C )both colourless
- D )both coloured
Actinoid ions are generally coloured. The colour of the ions depends upon the number of 5f − electrons. If 5f orbitals do not contain any electron or are exactly half filled, they will impart no colour.
Example :
Which of the following chemicals has no action on the elements of the actinoid series?
- A )Alkalies
- B )Nitric acid
- C )Boiling water
- D )Hydrochloric acid
The elements of actinoid series are basic in nature and so do not react with alkalies such as NaOH.
No comments:
Post a Comment